How Can Damaged Dna Be Repaired
Dna integrity is always under attack from environmental agents like skin cancer-causing UV rays. How do Dna repair mechanisms detect and repair damaged Deoxyribonucleic acid, and what happens when they neglect?

Because DNA is the repository of genetic information in each living prison cell, its integrity and stability are essential to life. Deoxyribonucleic acid, all the same, is not inert; rather, information technology is a chemical entity subject field to assault from the surround, and any resulting damage, if not repaired, will lead to mutation and perhaps disease. Perhaps the best-known instance of the link between environmental-induced DNA damage and illness is that of skin cancer, which can be caused by excessive exposure to UV radiations in the form of sunlight (and, to a lesser degree, tanning beds). Some other example is the damage caused by tobacco smoke, which can lead to mutations in lung cells and subsequent cancer of the lung. Beyond ecology agents, Dna is likewise subject to oxidative damage from byproducts of metabolism, such as free radicals. In fact, it has been estimated that an individual cell can suffer up to 1 million Deoxyribonucleic acid changes per day (Lodish et al., 2005).
In addition to genetic insults caused past the surround, the very process of DNA replication during cell sectionalization is decumbent to error. The rate at which Deoxyribonucleic acid polymerase adds incorrect nucleotides during DNA replication is a major cistron in determining the spontaneous mutation charge per unit in an organism. While a "proofreading" enzyme commonly recognizes and corrects many of these errors, some mutations survive this process. Estimates of the frequency at which human Deoxyribonucleic acid undergoes lasting, uncorrected errors range from 1 10 10-four to 1 x x-six mutations per gamete for a given cistron. A rate of 1 x 10-half dozen ways that a scientist would expect to find one mutation at a specific locus per ane meg gametes. Mutation rates in other organisms are often much lower (Table i).
One way scientists are able to guess mutation rates is by considering the rate of new ascendant mutations found at different loci. For example, by examining the number of individuals in a given population who were diagnosed with neurofibromatosis (NF1, a disease caused by a spontaneous—or noninherited—dominant mutation), scientists determined that the spontaneous mutation charge per unit of the gene responsible for this disease averaged i x 10-4 mutations per gamete (Crowe et al., 1956). Other researchers have constitute that the mutation rates of other genes, like that for Huntington's affliction, are significantly lower than the rate for NF1. The fact that investigators have reported different mutation rates for different genes suggests that certain loci are more prone to damage or error than others.
DNA Repair Mechanisms and Human Affliction

DNA repair processes be in both prokaryotic and eukaryotic organisms, and many of the proteins involved have been highly conserved throughout evolution. In fact, cells have evolved a number of mechanisms to detect and repair the various types of impairment that can occur to Dna, no affair whether this harm is acquired past the environs or by errors in replication. Because DNA is a molecule that plays an active and critical office in jail cell division, command of Dna repair is closely tied to regulation of the cell bicycle. (Recollect that cells transit through a cycle involving the Gone, S, Thousandtwo, and M phases, with DNA replication occurring in the S stage and mitosis in the M phase.) During the jail cell wheel, checkpoint mechanisms ensure that a cell'south Deoxyribonucleic acid is intact before permitting DNA replication and cell sectionalisation to occur. Failures in these checkpoints tin can lead to an aggregating of damage, which in turn leads to mutations.
Defects in DNA repair underlie a number of homo genetic diseases that affect a wide variety of body systems only share a constellation of mutual traits, virtually notably a predisposition to cancer (Table 2). These disorders include clutter-telangiectasia (AT), a degenerative motor condition caused by failure to repair oxidative damage in the cerebellum, and xeroderma pigmentosum (XP), a condition characterized by sensitivity to sunlight and linked to a defect in an of import ultraviolet (UV) damage repair pathway. In add-on, a number of genes that have been implicated in cancer, such as the RAD group, take besides been determined to encode proteins critical for Dna impairment repair.
UV Damage, Nucleotide Excision Repair, and Photoreactivation

Every bit previously mentioned, one important Deoxyribonucleic acid impairment response (DDR) is triggered by exposure to UV light. Of the three categories of solar UV radiation, only UV-A and UV-B are able to penetrate World'south atmosphere. Thus, these 2 types of UV radiation are of greatest concern to humans, peculiarly as standing depletion of the ozone layer causes higher levels of this radiations to reach the planet's surface.
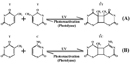
UV radiation causes ii classes of DNA lesions: cyclobutane pyrimidine dimers (CPDs, Effigy 1) and 6-four photoproducts (half-dozen-iv PPs, Figure 2). Both of these lesions distort Dna's structure, introducing bends or kinks and thereby impeding transcription and replication. Relatively flexible areas of the DNA double helix are virtually susceptible to damage. In fact, 1 "hot spot" for UV-induced damage is found within a commonly mutated oncogene, the p53 gene.
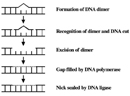
CPDs and 6-4 PPs are both repaired through a process known as nucleotide excision repair (NER). In eukaryotes, this complex process relies on the products of approximately 30 genes. Defects in some of these genes have been shown to cause the human affliction XP, too as other atmospheric condition that share a risk of pare cancer that is elevated about a thousandfold over normal. More specifically, eukaryotic NER is carried out by at least 18 poly peptide complexes via iv discrete steps (Effigy 3): detection of damage; excision of the section of Dna that includes and surrounds the mistake; filling in of the resulting gap by DNA polymerase; and sealing of the nick between the newly synthesized and older Deoxyribonucleic acid (Figure 4). In bacteria (which are prokaryotes), nonetheless, the process of NER is completed past only three proteins, named UvrA, UvrB, and UvrC.
Leaner and several other organisms also possess another mechanism to repair UV damage chosen photoreactivation. This method is often referred to as "calorie-free repair," considering it is dependent on the presence of light energy. (In comparing, NER and most other repair mechanisms are frequently referred to as "night repair," equally they do not require light as an energy source.) During photoreactivation, an enzyme called photolyase binds pyrimidine dimer lesions; in addition, a second molecule known as chromophore converts lite free energy into the chemical energy required to directly revert the affected area of DNA to its undamaged grade. Photolyases are constitute in numerous organisms, including fungi, plants, invertebrates such as fruit flies, and vertebrates including frogs. They do not announced to exist in humans, however (Sinha & Hader, 2002).
Additional DNA Repair mechanisms
NER and photoreactivation are not the only methods of DNA repair. For instance, base excision repair (BER) is the predominant mechanism that handles the spontaneous DNA impairment caused by free radicals and other reactive species generated by metabolism. Bases tin can become oxidized, alkylated, or hydrolyzed through interactions with these agents. For example, methyl (CHthree) chemical groups are frequently added to guanine to form 7-methylguanine; alternatively, purine groups may be lost. All such changes effect in abnormal bases that must be removed and replaced. Thus, enzymes known as DNA glycosylases remove damaged bases by literally cut them out of the DNA strand through cleavage of the covalent bonds between the bases and the sugar-phosphate courage. The resulting gap is then filled by a specialized repair polymerase and sealed by ligase. Many such enzymes are found in cells, and each is specific to certain types of base alterations.
Even so another form of DNA impairment is double-strand breaks, which are acquired by ionizing radiation, including gamma rays and 10-rays. These breaks are highly deleterious. In addition to interfering with transcription or replication, they can pb to chromosomal rearrangements, in which pieces of ane chromosome become attached to some other chromosome. Genes are disrupted in this procedure, leading to hybrid proteins or inappropriate activation of genes. A number of cancers are associated with such rearrangements. Double-strand breaks are repaired through one of 2 mechanisms: nonhomologous end joining (NHEJ) or homologous recombination repair (HRR). In NHEJ, an enzyme called DNA ligase Four uses overhanging pieces of Dna adjacent to the break to join and fill in the ends. Additional errors tin be introduced during this procedure, which is the case if a cell has not completely replicated its DNA in preparation for sectionalization. In contrast, during HRR, the homologous chromosome itself is used as a template for repair.
Mutations in an organism's DNA are a function of life. Our genetic lawmaking is exposed to a variety of insults that threaten its integrity. But, a rigorous system of checks and balances is in identify through the Deoxyribonucleic acid repair machinery. The errors that skid through the cracks may sometimes be associated with disease, merely they are also a source of variation that is acted upon by longer-term processes, such every bit evolution and natural selection.
References and Recommended Reading
Branze, D., & Foiani, G. Regulation of DNA repair throughout the cell cycle. Nature Reviews Molecular Cell Biology nine, 297–308 (2008) doi:10.1038/nrm2351.pdf (link to commodity)
Crowe, F. West., et al. A Clinical, Pathological, and Genetic Study of Multiple Neurofibromatosis (Springfield, Illinois, Charles C. Thomas, 1956)
Lodish, H., et al. Molecular Biological science of the Cell, 5th ed. (New York, Freeman, 2004)
Sinha, R. P., & Häder, D. P. UV-induced Deoxyribonucleic acid damage and repair: A review. Photochemical and Photobiological Sciences 1, 225–236 (2002)
How Can Damaged Dna Be Repaired,
Source: https://www.nature.com/scitable/topicpage/dna-damage-repair-mechanisms-for-maintaining-dna-344/
Posted by: knoxwhadn1945.blogspot.com


0 Response to "How Can Damaged Dna Be Repaired"
Post a Comment